Crystal water
The chemist wanted to check the water content of the crystallization of chromic potassium alum K2SO4 * Cr2 (SO4) 3 * 24 H2O, which took a long time in the laboratory. From 96.8 g of K2SO4* Cr2 (SO4) 3* 24 H2O, a 979 cm3 solution of base was prepared. Subsequently, the 293 cm3 of the stock solution, barium nitrate solution, and precipitate weighed 19.6 g.
How much crystal water, instead of 24 molecules, contains salt?
Additional data to compute:
M (K2SO4 * Cr2 (SO4) 3 * 24 H2O) = 998.2 g / mol, M(BaSO4) = 233.4 g / mol
M(K2SO4 * Cr2 (SO4) 3) = 566.2 g / mol, M (H2O) = 18 g / mol
How much crystal water, instead of 24 molecules, contains salt?
Additional data to compute:
M (K2SO4 * Cr2 (SO4) 3 * 24 H2O) = 998.2 g / mol, M(BaSO4) = 233.4 g / mol
M(K2SO4 * Cr2 (SO4) 3) = 566.2 g / mol, M (H2O) = 18 g / mol
Correct answer:

Tips for related online calculators
Do you have a linear equation or system of equations and are looking for its solution? Or do you have a quadratic equation?
You need to know the following knowledge to solve this word math problem:
Units of physical quantities:
Grade of the word problem:
Related math problems and questions:
- Salami
 We have six kinds of salami, six of which have ten pieces, and one of which has four pieces. How many ways can we distinctly choose five pieces of salami?
We have six kinds of salami, six of which have ten pieces, and one of which has four pieces. How many ways can we distinctly choose five pieces of salami? - Below 5
 Below is a collection of test scores from a class of 20 students. Make 2 histograms of the data. Choose your own horizontal scales as long as you have more than 4 cells in each histogram. 65 70 68 87 98 91 77 85 70 72 86 86 94 95 67 88 77 99 74 71
Below is a collection of test scores from a class of 20 students. Make 2 histograms of the data. Choose your own horizontal scales as long as you have more than 4 cells in each histogram. 65 70 68 87 98 91 77 85 70 72 86 86 94 95 67 88 77 99 74 71 - A contractor 2
 A contractor has to complete his contract in 92 days. 234 men were set to do the work, each of them works for 16 hours/day. After 66 days, 4/7 of the work is completed. How many additional men may be employed, so that the work may be completed in time, ea
A contractor has to complete his contract in 92 days. 234 men were set to do the work, each of them works for 16 hours/day. After 66 days, 4/7 of the work is completed. How many additional men may be employed, so that the work may be completed in time, ea - N percentille problem
 Here is a data set (n=117) that has been sorted. 10.4 12.2 14.3 15.3 17.1 17.8 18 18.6 19.1 19.9 19.9 20.3 20.6 20.7 20.7 21.2 21.3 22 22.1 22.3 22.8 23 23 23.1 23.5 24.1 24.1 24.4 24.5 24.8 24.9 25.4 25.4 25.5 25.7 25.9 26 26.1 26.2 26.7 26.8 27.5 27.6 2
Here is a data set (n=117) that has been sorted. 10.4 12.2 14.3 15.3 17.1 17.8 18 18.6 19.1 19.9 19.9 20.3 20.6 20.7 20.7 21.2 21.3 22 22.1 22.3 22.8 23 23 23.1 23.5 24.1 24.1 24.4 24.5 24.8 24.9 25.4 25.4 25.5 25.7 25.9 26 26.1 26.2 26.7 26.8 27.5 27.6 2
- Speed of Slovakian trains
 Rudolf took the train from the station 'Nitra' to 'Nové Zámky'. In the train timetables found train Os 5004 : km 0 Prievidza 14:25 4 Koš 14:30 14:31 9 Nováky 14:36 14:37 13 Zemianske Kostoľany 14:42 14:43 16 Bystričany 14:47 14:48 19 Oslany 14:51 14:51 23
Rudolf took the train from the station 'Nitra' to 'Nové Zámky'. In the train timetables found train Os 5004 : km 0 Prievidza 14:25 4 Koš 14:30 14:31 9 Nováky 14:36 14:37 13 Zemianske Kostoľany 14:42 14:43 16 Bystričany 14:47 14:48 19 Oslany 14:51 14:51 23 - Ivan rented
 Ivan rented a truck for one day. The base fee was $14.95, and he drove an additional charge of 73 cents for each mile. When he returned the truck, he had to pay $114.96. How many miles did he drive the truck?
Ivan rented a truck for one day. The base fee was $14.95, and he drove an additional charge of 73 cents for each mile. When he returned the truck, he had to pay $114.96. How many miles did he drive the truck? - Z-score calc
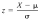 What is the Z Score for the following numbers: X is 16, and data for the population mean, and the standard deviation is 196, 193, 79, 30, 29, 81, 25, 21, 164 Level of difficulty = 2 of 2 Please format it to 2 decimal places.
What is the Z Score for the following numbers: X is 16, and data for the population mean, and the standard deviation is 196, 193, 79, 30, 29, 81, 25, 21, 164 Level of difficulty = 2 of 2 Please format it to 2 decimal places.
